Fill in a Valid Drug Screen Form
Document Sample
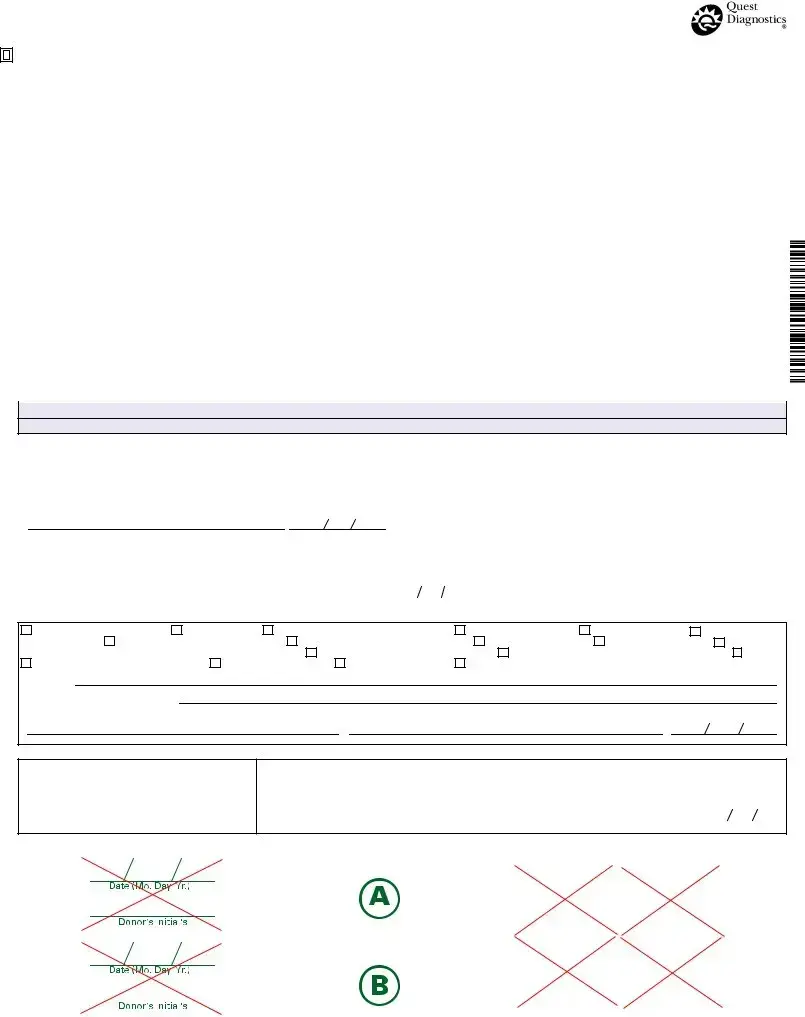
FEDERAL DRUG TESTING CUSTODY AND CONTROL FORM
SPECIMEN ID NO. |
|
STEP 1: COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE |
LAB ACCESSION NO. |
Quest, Quest Diagnostics, the associated logo and all associated Quest Diagnostics marks are the trademarks of Quest Diagnostics Incorporated. © Quest Diagnostics Incorporated. All rights reserved.
A. Employer Name, Address, I.D. No. |
|
|
B. MRO Name, Address, Phone and Fax No. |
||||||||||
|
|
|
|
|
|
|
|
|
|||||
C. Donor SSN or Employee I.D. No. _______________________________________________________________ |
|
|
|
|
|||||||||
D. SpecifyTesting Authority: HHS |
NRC |
DOT – Specify DOT Agency: FMCSA |
FAA |
FRA FTA PHMSA USCG |
|||||||||
E. Reason forTest: |
Random |
Reasonable Suspicion Cause Post Accident |
Return to Duty |
|
|||||||||
F. DrugTests to be Performed: |
THC, COC, PCP, OPI, AMP |
THC & COC Only |
Other (specify) ________________________________________________ |
||||||||||
G. Collection Site Name: |
|
|
|
|
|
Collection Site Code: |
|
|
|
|
|||
Address: |
|
|
|
|
|
|
Collector Phone No.: |
|
|
||||
City, State and Zip: |
|
|
|
|
|
Collector Fax No.: |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STEP 2: COMPLETED BY COLLECTOR (make remarks when appropriate) Collector reads specimen temperature within 4 minutes.
Temperature between 90° and 100° F? Yes No, Enter Remark |
Collection: Split Single None Provided, Enter Remark |
Observed, (Enter Remark) |
REMARKS
STEP 3: Collector affixes bottle seal(s) to bottle(s). Collector dates seal(s). Donor initials seal(s). Donor completes STEP 5 on Copy 2 (MRO Copy)
STEP 4: CHAIN OF CUSTODY - INITIATED BY COLLECTOR AND COMPLETED BY TEST FACILITY
|
I certify that the specimen given to me by the donor identified in the certification section on Copy 2 of this form was |
|
SPECIMEN BOTTLE(S) RELEASED TO: |
|||||||
|
collected, labeled, sealed, and released to the Delivery Service noted in accordance with applicable Federal requirements. |
Quest Diagnostics Courier |
||||||||
|
|
X |
|
|
|
|
|
FedEx |
||
|
|
Signature of Collector |
|
|
|
|
|
Other |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AM |
|
|
|
|
|
|
|
|
|
|
PM |
|
|
|
|
|
|
(Print) Collector's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|
Time of Collection |
|
|
Name of Delivery Service |
||
RECEIVED AT LAB OR IITF: |
|
|
|
|
|
Primary Specimen |
SPECIMEN BOTTLE(S) RELEASED TO: |
|||
|
X |
|
|
|
|
|
Bottle Seal Intact |
|
||
|
|
|
|
|
|
Yes No |
|
|||
|
|
Signature of Accessioner |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If No, Enter remarks |
|
|
|
|
|
|
|
|
|
|
in Step 5A. |
|
|
|
|
(Print) Accessioner’s Name (First, MI, Last) |
|
|
|
Date (Mo./Day/Yr.) |
|
|||
STEP 5A: PRIMARY SPECIMEN REPORT - COMPLETED BY TEST FACILITY
NEGATIVE |
POSITIVE for: |
Marijuana Metabolite ( |
6- Acetylmorphine |
Methamphetamine |
MDMA |
|
DILUTE |
|
|
Cocaine Metabolite (BZE) |
Morphine |
Amphetamine |
MDA |
|
|
|
PCP |
Codeine |
|
MDEA |
REJECTED FOR TESTING |
ADULTERATED |
SUBSTITUTED |
INVALID RESULT |
|
|
|
REMARKS:
Test Facility (if different from above):
I certify that the specimen identified on this form was examined upon receipt, handled using chain of custody procedures, analyzed, and reported in accordance with applicable Federal requirements.
X
Signature of Certifying Scientist |
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
STEP 5b: COMPLETED BY SPLIT TESTING LABORATORY
RECONFIRMED FAILED TO RECONFIRM - REASON ____________________________________________
___________________________________________ |
I certify that the split specimen identified on this form was examined upon receipt, handled using chain of custody |
||||||||||||||||||||||||||||
procedures, analyzed and reported in accordance with applicable Federal requirements. |
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Name |
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
___________________________________________ |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Signature of Certifying Scientist |
|
|
|
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Address |
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMB No.
PRESS HARD - YOU ARE MAKING MULTIPLE COPIES
Document Information
| Fact Name | Details |
|---|---|
| Form Title | This is the Federal Drug Testing Custody and Control Form. |
| Contact Information | The form includes a contact number: 800-877-7484. |
| Testing Authority | Testing may be conducted under authorities such as HHS, NRC, or DOT. |
| Collection Process | The collector must read the specimen temperature within 4 minutes of collection. |
| Specimen Handling | The chain of custody must be maintained throughout the testing process. |
Drug Screen - Usage Guidelines
Completing the Drug Screen form is a critical step in the testing process. Each section must be filled out accurately to ensure compliance with federal regulations. Following these steps will help streamline the process and minimize errors.
- Begin with the Collector or Employer Representative section. Fill in the Employer Name, Address, and I.D. No..
- Provide the MRO Name, Address, Phone, and Fax No..
- Enter the Donor SSN or Employee I.D. No..
- Select the Testing Authority from the options provided (HHS, NRC, DOT) and specify the DOT Agency if applicable.
- Indicate the Reason for Test by checking the appropriate box.
- List the Drug Tests to be Performed by selecting from the provided options.
- Fill in the Collection Site Name, Collection Site Code, Address, Collector Phone No., City, State, and Zip, and Collector Fax No..
- In the Collector section, check the specimen temperature within 4 minutes and indicate if it falls between 90° and 100° F.
- Specify the Collection type (Split, Single, None Provided) and make remarks as necessary.
- Affix the bottle seal(s) to the specimen bottle(s) and date the seal(s). Have the donor initial the seal(s).
- Complete the Chain of Custody section, certifying that the specimen was collected, labeled, sealed, and released in accordance with federal requirements.
- Record the Name of Delivery Service and ensure the specimen bottle(s) are released to the appropriate service.
- In the Primary Specimen Report, indicate whether the result is Negative or Positive for the specified substances.
- Complete the Split Testing Laboratory section if applicable, certifying the handling of the split specimen.
Common PDF Forms
Workmanship Warranty Example - This warranty highlights the dedication of MCS Roofing to quality installations and service.
In the process of buying or selling a mobile home, having the correct legal documentation is vital, and the Virginia Mobile Home Bill of Sale form is a key component in this transaction. This form, which acts as proof of sale, includes important information such as the details of the buyer and seller, a description of the mobile home, and the sale price. For more information on this essential document, you can visit mobilehomebillofsale.com/blank-virginia-mobile-home-bill-of-sale. Ensuring accuracy in these details helps protect the rights of both parties and streamline the ownership transfer process.
Citizens Roof Certification Form - Document spalling on walls to identify signs of broader deterioration within the structure.
Dos and Don'ts
When filling out the Drug Screen form, it’s important to be thorough and accurate. Here are five key things to do and not do:
- Do: Provide accurate information about your employer, including name and address.
- Do: Clearly specify the reason for the test, such as pre-employment or random.
- Do: Ensure that all required fields are completed, including your Social Security Number or Employee ID.
- Do: Confirm the temperature of the specimen within the specified time frame.
- Do: Sign and date the form where required to validate the information provided.
- Don't: Leave any sections blank; incomplete forms may lead to delays.
- Don't: Provide false information or misrepresent your identity.
- Don't: Forget to initial the bottle seals; this step is crucial for maintaining chain of custody.
- Don't: Use any unapproved abbreviations or shorthand that could confuse the reader.
- Don't: Neglect to follow up if you notice any discrepancies after submission.
Common mistakes
-
Inaccurate Personal Information: Filling out the donor's name, Social Security Number (SSN), or employee ID incorrectly can lead to significant delays. Ensure all information matches official documents.
-
Missing Testing Authority: Not specifying the testing authority can cause confusion. Always select the appropriate agency, whether it’s HHS, NRC, or DOT, and indicate the specific DOT agency if applicable.
-
Incorrect Reason for Test: Choosing the wrong reason for the drug test can affect the process. Make sure to select the accurate option, such as pre-employment, random, or post-accident.
-
Failure to Document Collection Site: Omitting the collection site name and address can create complications. It is essential to provide complete details of where the specimen is collected.
-
Neglecting to Sign and Date: Forgetting to sign or date the form can lead to rejection. Always ensure that the collector and donor sign and date the appropriate sections.
